
What is Soap made of and how does it work?
We have all grown up being told to wash with soap but how many of us have considered why and how soap actually works?
When was soap invented?
There is evidence that humans have been using soap for nearly 5000 years and it’s safe to assume that back then they didn’t strictly have the scientific ability to see bacteria and understand that it could be washed away with soap. It is more likely they were focussed on cleaning dirt of the skin and didn’t realise the anti-bacterial benefits that soap provided.
How is soap made?
While there are several methods for making soap, the two most common methods are the cold and hot processes. Both methods involve combining fats or oils with an alkali (such as sodium hydroxide) to create soap through a process called saponification. Here's an overview of these two main soap-making methods:
Cold Process Soap Making:
- Mix the lye with water, carefully following safety precautions.
- Heat the oils to a specific temperature and then combine them with the lye solution.
- Stir the mixture until it reaches trace, which is the point where the soap has thickened and the lye and oils have emulsified.
- Add any desired fragrances, colours, or additives.
- Pour the soap into moulds and let it cure for several weeks, allowing the saponification process to complete.
Hot Process Soap Making:
- Combine the oils and lye solution, and heat the mixture in a slow cooker or on the stove.
- Stir the mixture continuously as it cooks. The heat speeds up the saponification process.
- Once the soap reaches a mashed-potato-like consistency (known as "gel phase"), add any desired fragrances, colours, or additives.
- Spoon the soap into moulds immediately, as hot process soap is ready to use right away. However, allowing it to cure for a few weeks can still improve the quality.
Is the Hot or Cold process better for making soap?
The main advantage of the hot process is that it has a shorter “curing” time. This means you can use the soap as soon as it is hardened whereas the cold process requires you to let the soap cure for weeks.
That said soaps made using the hot process don’t tend to look as clean and smooth as soaps made using the cold process.
This means that if you were making a soap at home for personal use then you might favour the hot process for it’s speed but if you were thinking of making soaps for sale (or for a guest bathroom) then the cold process might result in a higher quality finish.
How does soap work?
Everyone knows that soap has antibacterial properties but let’s now look at how that works in principle:
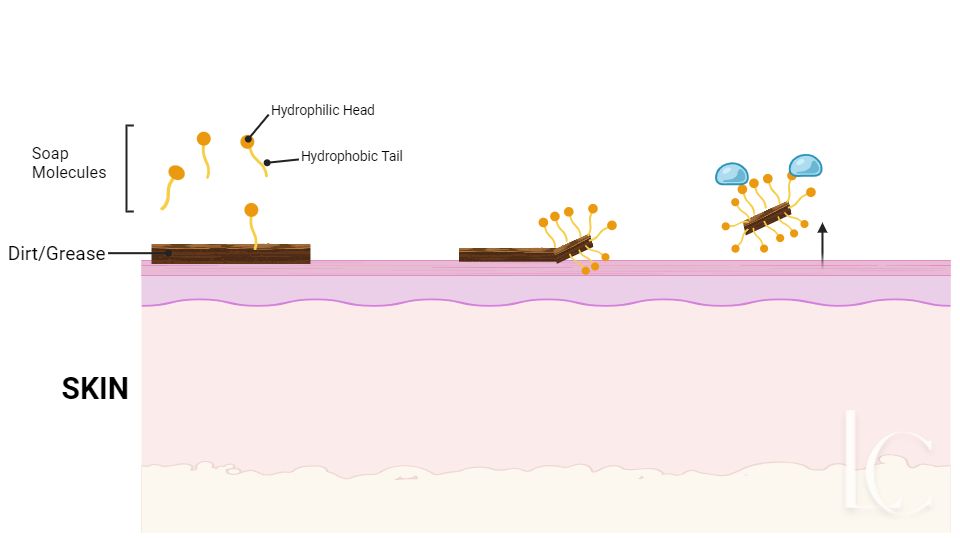
Soap molecules look like this. They have a “head” which is attracted to water and a “Tail” which is repelled by water.
Dirt is also hydrophobic; it doesn’t like water. When you apply soap to your skin, the hydrophobic tails are attracted to the dirt and stick to it. Then, when you run your hands under water, the hydrophilic head gets attached to the water and this has the effect of dragging the dirt off your skin as the head carries the tail, which is still attached to the dirt, away.
Are all soaps natural?
No. We have described only natural soaps thus far which are made using alkalis and fats. That said there are also synthetic soaps on the market.
Synthetic soaps tend to contain parabens, synthetic fragrances, artificial colours, and Sodium Laureth Sulfate (SLS). SLS is famous for being the thing that makes a soap foamy but it is also famous for irritating skin and drying it out. People who suffer from eczema always seek out bath products that don’t contain any SLS.
What other artificial ingredients are found in soaps?
It’s important to remember that soaps will sometimes combine both natural and artificial ingredients. Other things to look out for on the ingredients list of a soap are:
- Artificial colouring
These are often a colour followed by a number. E.g. Yellow 5. These are known as FD and C colours.
- Fragrance Oils
Not to be confused with essential oils, which are natural. Fragrance oils are a cheap way of companies getting soaps to smell good.
- Lathering Agents
Aside from Sodium Laureth Sulfate (SLS) companies also use agents like Cocamide DEA, MEA and TEA. These create an artificial foamy lather. This lather might feel nice but can dry out the natural oils on the skin and cause irritation or itchiness.
Is it better to use hand sanitiser or soap?
Hand Sanitiser does a similar job to soap but in a different way. Hand sanitiser breaks apart a bacterial or viral cell and this has the effect of killing it. That is why hand sanitisers tend to need a large percentage of alcohol to be effective.
What is the difference between hand sanitiser and soap?
Whilst both will kill bacteria and viruses on the skin, we tend to recommend using soap at Landys Chemist for a few reasons:
- Visibility
You can see a soap on your skin and thus make sure it is covering every part of your hands. You can also scrub to make sure it gets any dirt under your nails. It is harder to see if an alcohol based hand sanitiser has effectively been on every part of your hands and the alcohol evaporates off your skin as you rub it in meaning that you might think you’ve covered a part of your hand when really you didn’t.
- Comfort
Soaps tend to contain an ingredient called glycerin. Glycerin is a natural agent that keeps skin hydrated and smooth. A lot of synthetic soaps also inject glycerin in because of its hydrating properties. There are very few hand sanitisers that contain glycerin and so they can be more drying and irritating to hands.







